Extracellular degraders enter the fray
12th January 2024
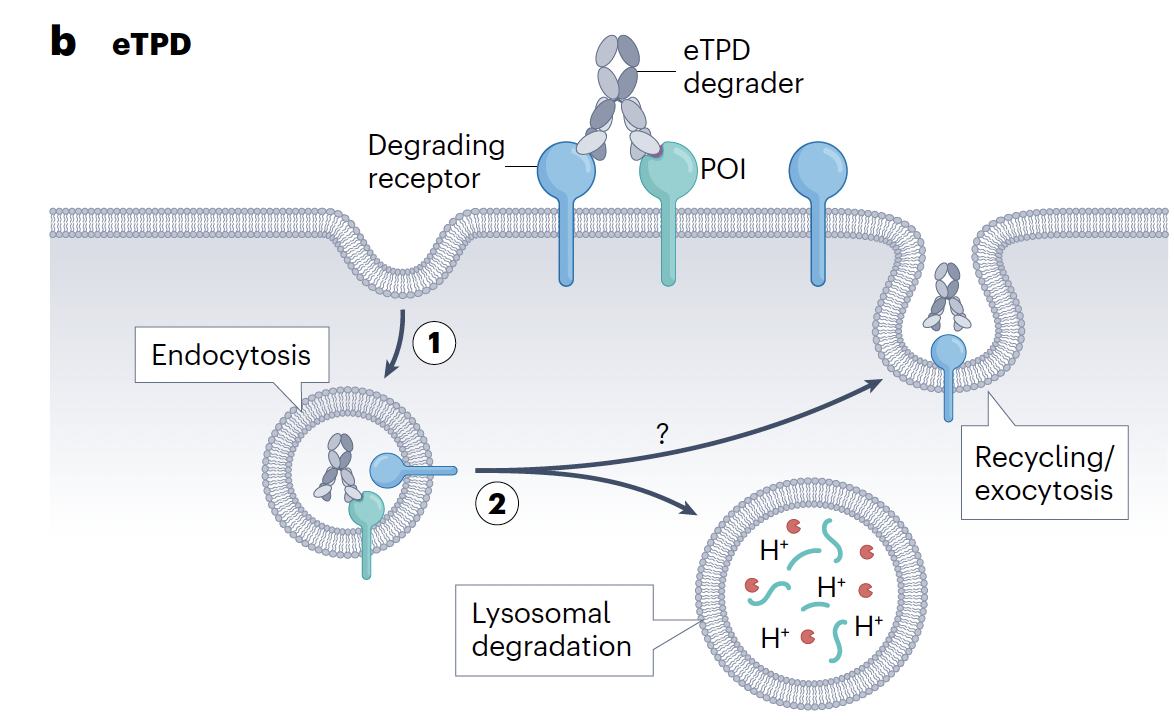
Designing drugs to degrade intracellular proteins using PROTACs, glues etc has advanced at a dizzying speed with the first drug approvals now within sight but degrading extracellular or membrane-bound proteins has proved a somewhat tougher nut to crack.
A useful survey of the range of approaches to tackle these proteins recently appeared in Nature Reviews Drug Discovery, authored by Jim Wells (& his PhD sudent Kaan Kumru) who knows this space very well having published extensively and also co-founded TPD biotech EpiBiologics.
It's remarkable to note that, despite the huge success of antibody-based therapeutics over the last 30+ years, marketed agents only target less than 40 proteins leaving the majority of extracellular target space still requiring effective ways to modulate, with degradation offering interesting therapeutic possibilities.
Can everything we have learned with PROTACs & glues for intracellular degradation be directly transferred to extracellular degraders? Well, partially, as the same ternary complex-based mechanistic paradigm underpins activity though there are important differences most notable being that disclosed extracellular degradation mechanisms most frequently rely on antibody-based therapeutics (except ASGPR mechanisms) rather than small molecules. Also, the catalysis seen with PROTACs is not always reproduced with extracellular degraders where the therapeutic often goes down with the ship and is also degraded. It’s also notable that degrading extracellular or membrane-bound proteins (the authors term this eTPD) most commmonly uses the lysosomal route of degradation rather than the proteasome which may lead to slower kinetics. Finally, while VHL & CRBN-based mechanisms currently dominate intracellular degrading strategies, there remain a plethora of potential eTPD mechanisms without a clear preferred method yet to emerge. This may be simply a temporal issue as eTPD research applied to drug discovery is perhaps 5-10 years behind its intracellular cousin.
Here's a quick summary of a few salient points of the various approaches:
Sweeping antibodies were perhaps the first ePTD agents with satralizumab (clearing IL-6 receptor) approved in 2020. They are simple “mono-specific” antibodies which bind and internalize extracellular POIs which are then degraded in the lysosome after release using a pH-switchable process (which can be tricky to design) while the antibody is recycled via FcRn. May be good for highly abundant POIs.
Hijacking the lysosomal pathway: Using bispecific agents vs POI & natural cell surface receptors as gateways to the endolysosomal pathway which currently has two main flavours:
Using the cation-independent mannose 6-phosphate receptor (CI-M6PR, mainly expressed in immune cells) via use of large glycan ligands & biologics. Works well but may not be catalytic and there is some suggestion that the mechanism may be better for membrane-associated rather than soluble POIs.
Alternatively, may internalize by engaging ASGPR, a liver-specific glycan receptor which can be recruited by small molecule ligands giving the potential for all-small-molecule eTPD therapeutics. Reported to be faster, degrading abundant proteins within 2h although again may not be catalytic.
Amongst TPD biotechs, Lycia (LYTACs), GlycoEra (G-LyTACs), Biohaven (MoDE) and Avilar (ATACs) are active in this space.
Engaging cell surface E3 ligases in PROTAC-like mechanisms: Antibody-based chimeras (known as AbTACs or PROTABs) are bispecifics developed to target the intramembraneous E3 ligases such as RNF43 (& other RNF family ligases) or ZNRF3. Tandem IgG constructs may give increased Dmax potentially suggestive of the use of clusters of E3 ligases. Other, single domain agents based on VHH domains can also be used to recruit RNF family E3s. Amongst TPD biotechs, EpiBiologics & Indupro are active in this space. Genentech have also published here.
Using cytokines or other recycling receptors: Bispecific cytokine receptor-targeting chimeras (KineTACs) have been designed to recruit cytokine recycling receptor CXCR7 by incorporation of CXCR7 ligand CXCL12 in a mechanism which is likely catalytic. KineTACs may also be useful for uptake of soluble targets eg VEGF. Integrin receptor binders (eg cyclic RGD peptides) can also be used in bifunctional molecules to internalise and degrade via avb3 receptors with PoC shown with eg PDL1.
So there’s a wide range of potential approaches – which ones reach the clinic and market first remains to be seen but it’s likely some will float to the top and others may not translate so well. Reports of more potent effects mainly focus on cell surface receptors so far with degradation of soluble proteins appearing to be slightly less potent or optimised yet. One class of membrane-bound target which is still proving tough for TPD is ion channels – I’m not sure I can recall any good examples of potent degradation of these (though groups are using bifunctionals to stabilize them of course).
Interestingly, across the range of eTPD mechanisms, Dmax values are typically in 60-80% range so not as high as PROTACs where 90%+ is routinely achievable. This may be due to a slower intrinsic degradation rate of eTPD mechanisms due to the slower processing through the endolysosomal pathway or else the low expression level of the receptors used relative to POI levels - a number of eTPD studies show the importance of a higher ratio of expression of the receptor used to modulate degradation relative to POI.
We await further progress and disclosures with interest!
We need your consent to load the translations
We use a third-party service to translate the website content that may collect data about your activity. Please review the details in the privacy policy and accept the service to view the translations.

