Drug targeting remains a hot topic to increase drug efficacy and reduce the potential for undesired systemic effects. In TPD space, much energy has been expended looking for tissue-specific ubiquitin E3 ligases which would give degradation of intracellular targets in specific cells with high expression of the E3 only. This has proved extremely challenging as there are very few E3s with suitable expression patterns and of those, persuading them to be recruited by bifunctional molecules as easily as CRBN or VHL to give potent cellular degradation has been very elusive indeed.
Degrading extracellular targets using systemically-delivered LYTACs through a lysosomal route faces similar challenges as identification of cell-type selective lysosomal trafficking receptors is also non-trivial. Taking a step back though, the concept of degrading in certain cell types only may not even be most relevant – instead using spatially restricted therapies which can degrade target proteins in multiple, relevant cell types in a defined, diseased organ or tumor microenvironment may be therapeutically more advantageous.
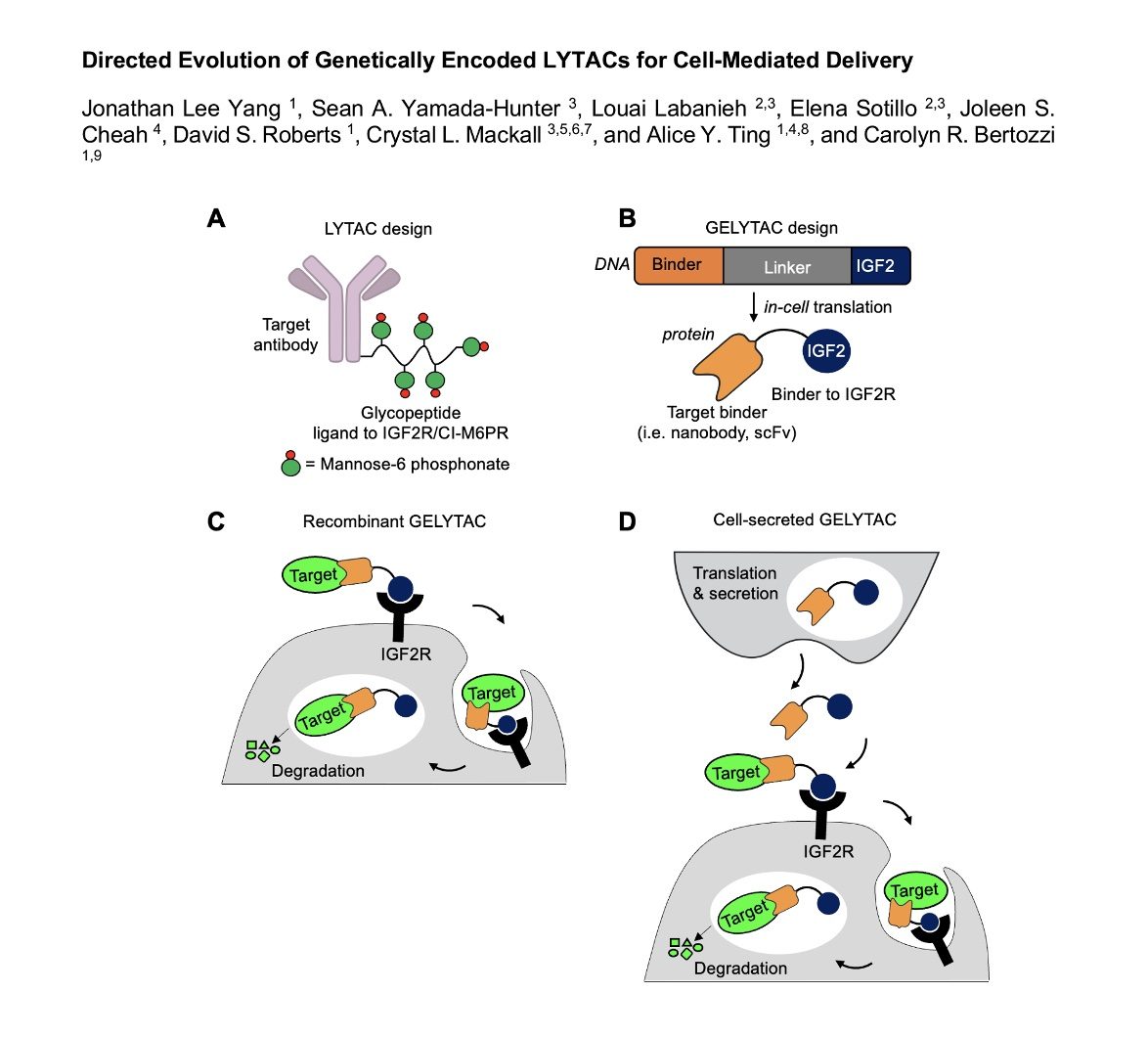
To address this, Carolyn Bertozzi’s team at Stanford have now developed genetically-encoded LYTACs – GELYTACs (See BioRXiv link here). A bifunctional GELYTAC comprises a POI binder in the form of a nanobody or scFv linked to an IGF2R ligand in order to be picked up by cells to enter the lysosomal pathway. This therapy can be produced recombinantly then dosed systemically or, to introduce spatial delivery and degradation, T cells can be engineered to express the GELYTAC constructs allowing the therapeutic protein to be secreted wherever the T cell is directed to in the body (via eg CAR-T strategies).
The initial proof of principal used a recombinant GELYTAC based on native IGF2 linked to an mCherry-binding protein which worked well to internalize a probe substrate mCherry. A further tweak in activity was neatly done via directly evolution using error prone PCR on the IGF2 protein region of the GELYTAC to give a higher affinity, triple mutant IGF2R binder which translated into a 6 fold improvement in cellular uptake efficacy of the evolved GELYTAC.
Fortunately, things also work in a cell setting with an engineered KEK 293 “donor” cell able to secrete GELYTAC which is then taken up by IGF2R-expressing K562 “receiver” cells leading to partial degradation of exogenously added mCherry. HEK cells expressing GELYTACs designed to bind to TGFb or IL-6 also showed good uptake by K562 cells. Finally, to bridge towards use in CAR-T backgrounds, the construct could be successfully transfected into primary human T cells which then allowed substrate uptake in receiver cells in co-culture (though subsequent degradation of protein was not quantified).
Furthermore, as the final GELYTACs are still small proteins (20-30kD), they would be expected to have modest plasma half-lives meaning that any therapeutic that diffused away from the desired site of action would likely be cleared and less likely to mediate off-tissue effects.
Lots of good concepts here and you could imagine next generation CAR-T cells expressing proteins which give selective degradation of proteins in specific locations combined with all the other tricks that can be played with cellular, genetically-encoded agents such as environment-sensitive logic gate expression switching etc
CAR-T development is not in itself always straightforward and with so many variables around specificity of localisation of cells, GELYTAC expression level, rate of uptake by degrading cells and level of actual degradation in the face of protein resynthesis rate to grapple with, design & optimisation may prove to be challenging but, if everything works to plan, there could be some exciting therapeutic options which come out of this.
We need your consent to load the translations
We use a third-party service to translate the website content that may collect data about your activity. Please review the details in the privacy policy and accept the service to view the translations.

