Degraders join the ADC party
6th November 2023

Everyone seems to want an antibody-drug conjugate (ADC) therapy right now with big pharma doing deals for clinical phase and earlier assets and technologies at an increasing rate.
ADC drugs usually marry a tumor-selective antibody to a cytotoxic payload but several TPD companies think that using a protein degrading drug in place of the cytotoxic could give agents with improved activity and therapeutic index. Like an ADC but actually termed a DAC, degrader antibody conjugates promise to combine the highly potent cell killing activity of TPD drugs (bifunctionals or glues) with exquisite tumor targeting delivered by the antibody. Orum Therapeutics are perhaps the leaders in this space and recently announced a deal with BMS who will acquire their clinic-ready GSPT1-aCD33 DAC known as ORM-6151.
GSPT1 is a well-known glue target with potential utility in AML, ALL and some solid tumors with several molecules in the clinic or approaching it (eg CC-90009, MRT-2359). The powerful anti-leukaemic effects of GSPT1 degraders can however sometimes be accompanied with tolerability concerns requiring careful management of, often heavily pretreated and unwell, patients. In particular, hypocalcemia and hypotension have been noted as more prevalent, potentially on-target, treatment-emergent adverse events.
To address these potential tolerability concerns, GSPT1 degraders have been conjugated to antibodies, in the case of ORM-6151, to an aCD33 Ab (NB Orum have also conjugated a GSPT1 glue to aHER2/3 Ab to give ORM-5029, currently in PhI for treatment of breast tumors).
Optimising ADCs is now becoming a better understood field though an often complex route involving optimising drug loading, desired linker & cleavage profile as well as physicochemical properties and other factors can still be time-consuming. Preparing and optimising degrader antibody conjugates has built on this experience with low molecular weight glues particularly well suited to DAC work due to their high intrinsic potency and favourable chemical properties (though many groups are also advancing DACs based on PROTACs).
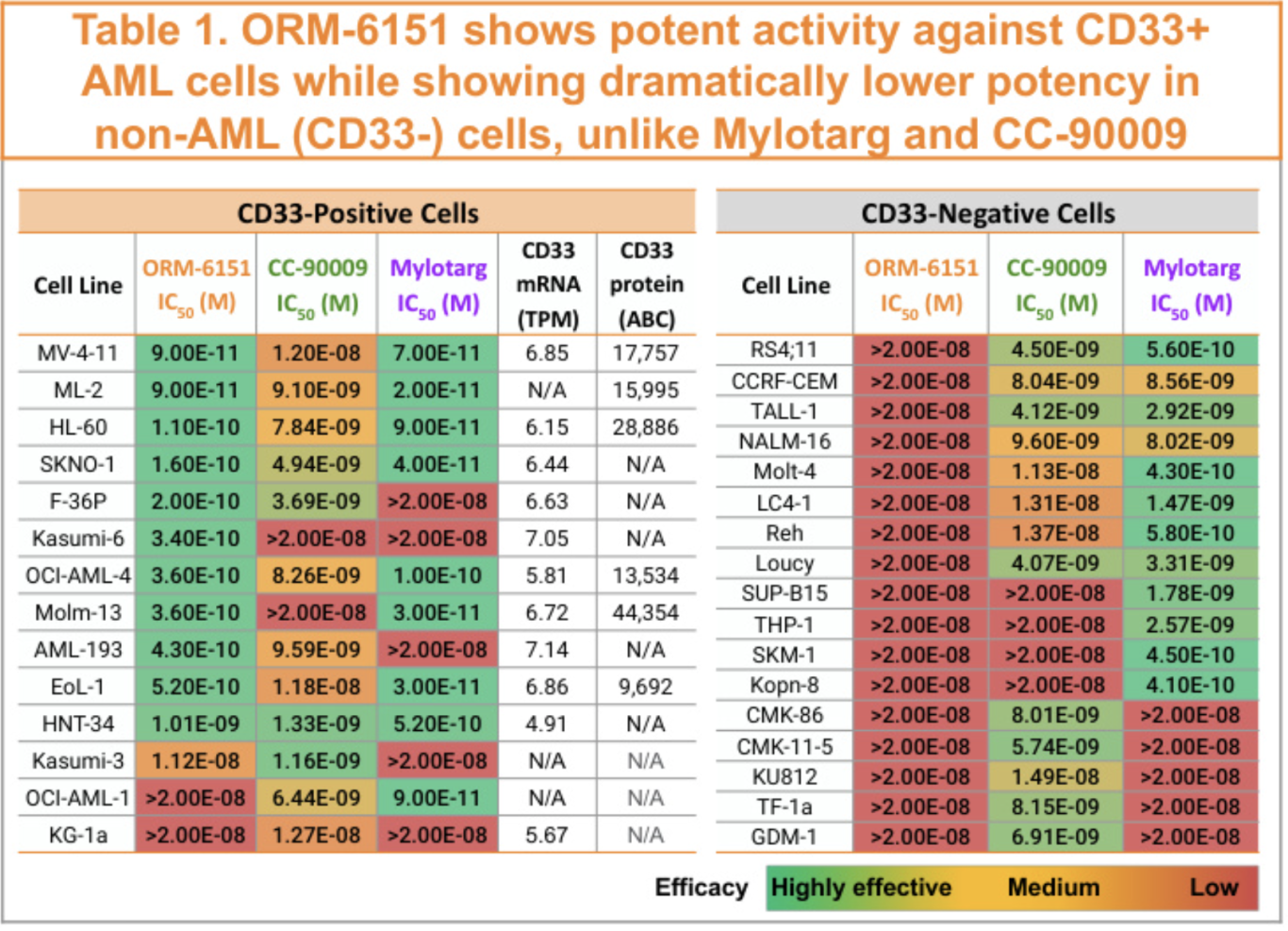
The payoff for taking the time to design a DAC should be high potency in cells expressing the targeting antigen (CD33) with minimal cytotoxicity in cells lacking this marker. Fortunately, ORM-6151 demonstrates this profile nicely (see table below taken from Orum's 2022 ASH poster) with sub-nM activity in almost all CD33+ve cells and no activity across a panel of CD33-ve cells. Contrast this to the bare GSPT1 degrader CC-90009 or Mylotarg (Gemtuzumab Ozogamicin, an enediyne cytotoxin-aCD33 conjugate) which show a less CD33+ve-selective effect across the cell panel - the hope is that the clinical tolerability profile of ORM-6151 may be superior.
BMS, who after all, through their Celgene heritage, know more about molecular glues than most companies, clearly like this idea also and have been willing to pay $100M upfront to acquire the ORM-6151 programme (with a further $80M on offer for further milestones).
We wait with interest whether the pre-clinical advantages shown over prior GSPT1-based therapies are also borne out in AML patients who need new options for an under-served tumor type which has seen precious few, broadly applicable (beyond specific mutation types), new treatment options in recent years.
We need your consent to load the translations
We use a third-party service to translate the website content that may collect data about your activity. Please review the details in the privacy policy and accept the service to view the translations.

