Another undruggable target falls? Let's wait and see
16th November 2023
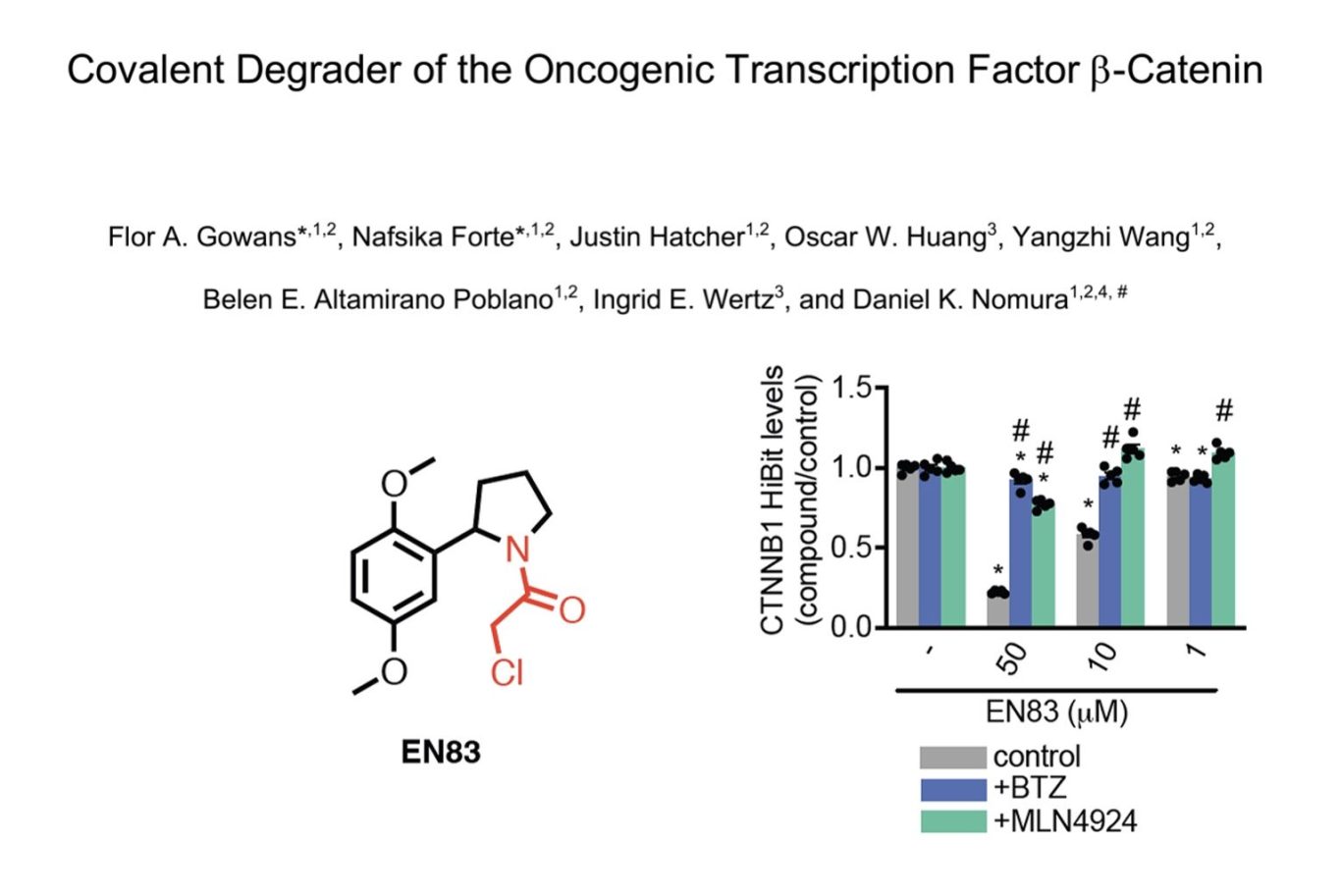
For a long time the holy trinity of undruggable oncogenic targets were KRAS, c-myc & beta catenin. KRAS has famously shed it's undruggable tag and c-myc, with its lack of tertiary structure and fleeting nature is still proving tricky. Beta-catenin has also so far proved elusive leading drug discoverers to look elsewhere in the pathway like tankyrase inhibitors or TCF PPI inhibitors but has Dan Nomura's group found a new, more direct way in?
They report monovalent degraders of beta-catenin in their latest BioRXiv preprint using the familiar range of haloacetamide covalent warheads which appear frequently in work from this group. (see also related efforts to degrade c-myc here, recruit DDB1 here and recruit E2 ligases here).
Screening around 2000 covalent fragments (chloroacetamides and acrylamides) showed around 1% lowered beta-catenin levels using a HEK HiBit assay though only a single hit, EN83, did this in a proteasomally-dependent manner with a modest DC50 ~10uM. NB This shows how beta-catenin levels can apprarently be sensitive to up to 1% of ligands through a range of undefined mechanisms - a cautionary observation for any phenotypic assay to discover apparent protein degraders) .
The mechanism of action of EN83 may however be more complex as significant effects on downstream signalling were seen (Fig 1g) at concentrations down to 10nM (where no degradation was observed) suggesting activity independent of degradation may also be occurring. Similarly, in beta-catenin-dependent tumor lines, downregulation of beta-catenin was only seen transiently with recovery at 24h, showing the importance of running time courses, especially with proteins known to have fast turnover rates.
Further MoA detail came using activity-based probes which showed that EN83 can covalently bind to at least 4 cysteine residues on beta-catenin (of which 3 appear to be linked to degradation efficacy) and in doing so appears to destabilize the protein as shown using a CETSA-type assay, inferring a denaturing profile. (Fig 3c,d). Coupled to the fact that selectivity profiling showed over 100 other proteins modified by EN83, this mode of action may be somewhat pleiotropic, involving destabilization of a range of substrates - introducing selectivity may be a significant challenge in order to optimise these molecules towards clinical use.
Pleasingly, this work also showed that the drug discovery-unfriendly chloroacetamide warhead could be replaced with the slightly less medchem-unpleasant oxophenylbutenamide to retain proteasomally-dependent degradation but clearly much more work is required to define if these covalent fragments operate through an optimisable, selective mechanism likely to give acceptable beta-catenin-specific effects with good tolerability. Should these molecules possess general denaturing activity, even if this is unrelated to the desired on-target effects, it will complicate their optimisation significantly.
Once again Dan's group has shown some very interesting observations using the power of covalent fragments which can clearly modulate protein function in many, diverse ways. Will this be the starting point for the development of a new wave of direct, beta-catenin-based antitumor therapies? Let's wait and see.
We need your consent to load the translations
We use a third-party service to translate the website content that may collect data about your activity. Please review the details in the privacy policy and accept the service to view the translations.

